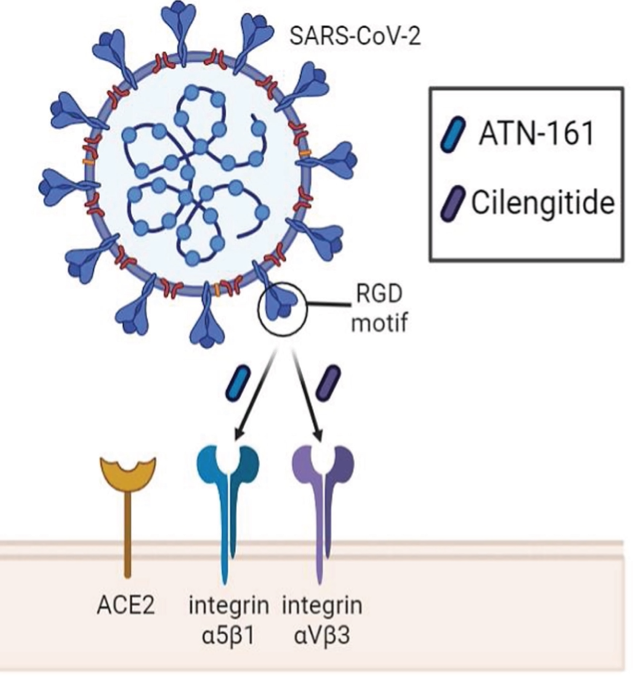
Integrins as Therapeutic Targets for SARS-CoV-2
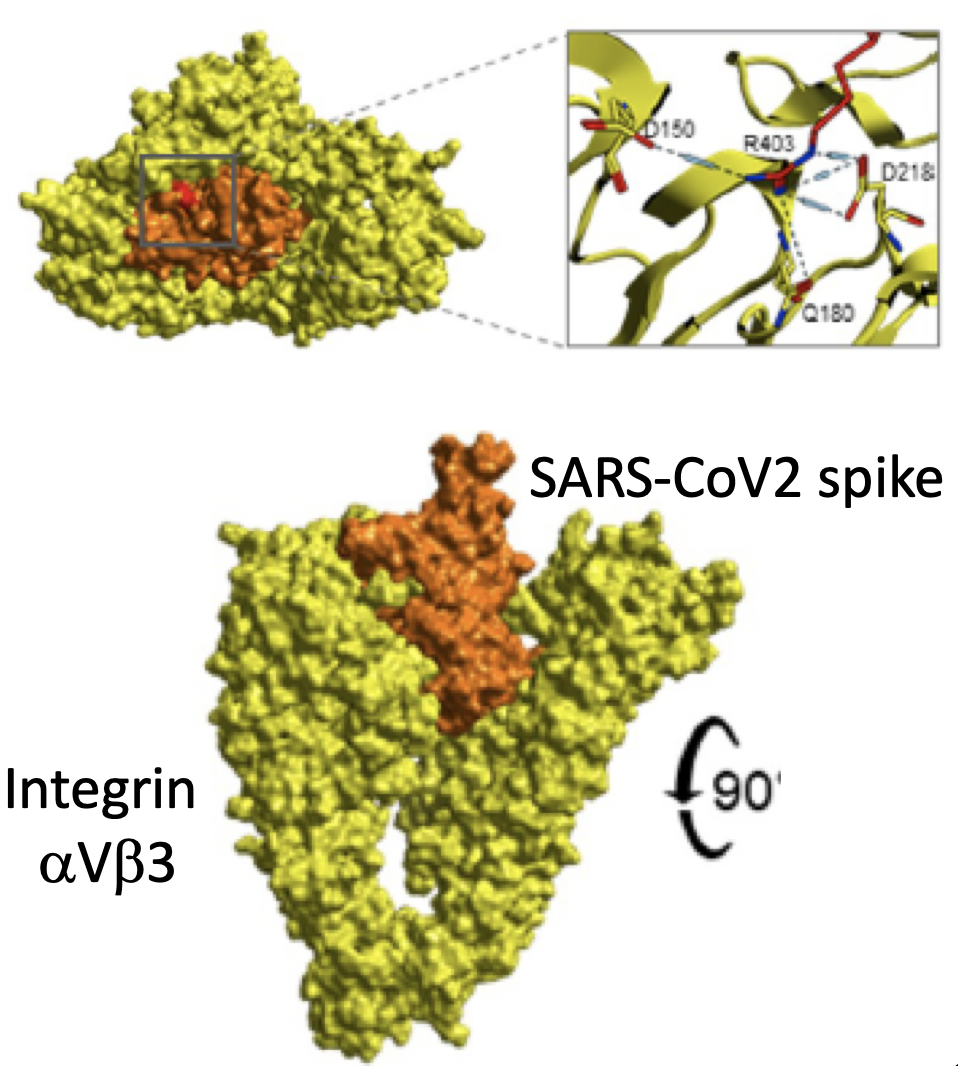
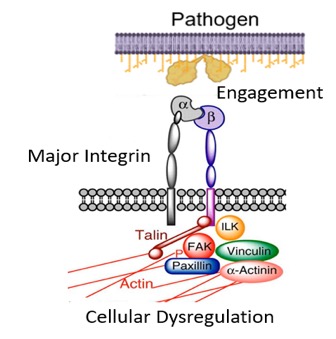
While the relationship between the spike protein of SARS-CoV-2 and the angiotensin converting enzyme 2 (ACE2) receptor has been readily established, the S1 subunit also contains a solvent-exposed arginine-glycine-aspartic acid (RGD) binding motif that is predominantly recognized by integrins.
Download Now